Background: Heavy menstrual bleeding (HMB) negatively impacts quality of life directly and indirectly due to symptoms associated with resultant iron deficiency (ID) and iron deficiency anemia (IDA). Antifibrinolytics, to decrease blood loss, and iron supplementation, to replace deficits, should be cornerstones of HMB management. Despite an abundance of evidence supporting their effectiveness and safety, clear, consistent guidance on treating HMB and secondary ID/IDA is lacking which renders access to care suboptimal and inequitable.
Objective: To identify barriers and facilitators to health care provider (HCP) access of oral antifibrinolytic agents and iron supplementation for patients with HMB globally.
Methods: We conducted a global survey on HCPs' experience in accessing antifibrinolytics, oral and intravenous (IV) iron. Participants were identified via snowball sampling using professional connections and/or social media. Descriptive analysis was used. Given the voluntary nature of our survey, the total number of responses varied with each question. This study was approved by the institutional research ethics board.
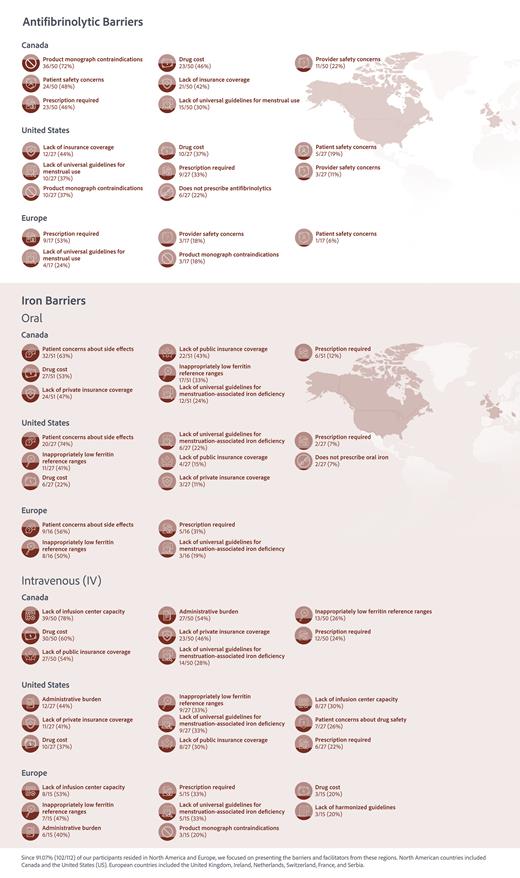
Results: A total of 113 HCPs responded, and practiced in North America (74%), Europe (17%), Central and South America (2%), Middle East (2%), Africa (1%), and Australia (1%). Specialties included obstetrics and gynecology (41%), hematology (21%), family medicine (16%), internal medicine (11%), pediatrics (9%), and maternal fetal medicine (1%). See Figures 1 and 2 for antifibrinolytic and iron barriers by region, respectively.
Antifibrinolytic Top Global Barriers: survey respondents reported product monograph contraindications (50/103, 49%), prescription requirement (46/103, 45%), lack of insurance coverage (34/103, 33%), patient safety concerns and drug cost (both 33/103, 32%).
Antifibrinolytic Top Global Facilitators: HCPs reported access to websites for knowledge translation (35/85, 41%), readily available education materials (27/85, 32%), and drug access navigators (16/85, 19%).
Oral Iron Top Barriers: HCPs reported patient concern regarding adverse effects of oral iron (64/101, 63%), inappropriately low ferritin reference ranges (38/101, 38%), drug cost (36/101, 36%), lack of private insurance coverage (27/101, 27%), and lack of public insurance coverage (26/101, 26%).
Oral Iron Top Facilitators: HCPs reported readily available education materials (43/82, 52%), websites for knowledge translation (33/82, 40%), and drug access navigators (19/82, 23%).
IV Iron Top Barriers: HCPs reported lack of infusion center capacity (58/101, 57%), drug cost (51/101, 51%), administrative burden (50/101, 50%), lack of public insurance coverage (40/101, 40%), and lack of private insurance coverage (37/101, 37%).
IV Iron Top Facilitators: HCPs reported publicly funded infusion clinics (55/99, 56%), private infusion clinics (29/99, 29%), readily available education materials (25/99, 25%), websites for knowledge translation (23/99, 23%), and drug access navigators (21/99, 21%).
Conclusions: Despite geographical variability in funding of health services and medications, there was a surprising consistency in the top reported barriers and facilitators to antifibrinolytic and iron access among international survey respondents. A key limitation is the predominant representation from North American and European countries. Further research in other regions is needed to allow us to gauge and address global barriers and facilitators more comprehensively.
Disclosures
Weyand:Pfizer: Research Funding; Bayer: Other: Consulting; Novo Nordisk: Other: Consulting, Research Funding; Spark: Other: Consulting; Genentech: Other: Consulting; Sanofi: Other: Consulting, Research Funding; Takeda: Other: Consulting, Research Funding. Sholzberg:Pfizer: Honoraria, Research Funding; CSL Behring: Research Funding; Octapharma: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal